| |
|
|
|
|
|
| Without A Trace |
Author: Kevin Dunn
Wednesday, May 3, 2017 |
|
With all the hoopla over fake news this year, I thought I would devote this month's column to “fake” or “false trace.” But to get there, we need to review the how and why of authentic trace. While the cold process dates back to an English patent in 1622, I have found no mention of the trace phenomenon earlier than Adolph Ott's The Art of Manufacturing Soap and Candles (1867). In the chapter on the cold process, he says, “For stirring [the raw soap], a broad paddle of boxwood must be used, having sharp edges at its lower end, rounded at its upper end, so that it may be the more easily handled. The paddling should be continued until a ring drawn with the spatula may be recognized.” Soapmakers have been seeking that mysterious ring ever since. But why?
Trace is not a requirement for turning oil to soap. In fact, you don't even have to mix oil and lye to get them to react. I once poured some 33% lye into a vial followed by a layer of olive oil. I carefully weighed the relative amount of oil and lye to correspond to the proper saponification value, but I did not mix them. The dense lye sat at the bottom of the vial, and the oil floated on top. Weeks later I noticed a layer of soap at the interface, and months later the vial was filled with soap. The lesson I took from this is that lye will eventually find any available oil and turn it to soap. Eventually. As a matter of practice, however, we mix oil and lye to speed up the reaction and produce a uniform product. When you mix oil and lye with a stick blender, an emulsion forms, consisting of lye droplets suspended in oil. If the oil and lye did not react, two neighboring lye droplets would combine to form a larger droplet. The larger droplets would coalesce into even larger droplets, and these dense droplets would settle to the bottom of the container, forming a layer of lye. Eventually, a layer of soap would form at the boundary between oil and lye, and in the distant future the oil would turn to soap, just as it did in the experiment above. But fortunately for us, we don't have to wait for “eventually.” When the oil/lye mixture is emulsified, the area of contact is increased enormously, and a layer of soap forms at the surface of each lye droplet. This layer of soap prevents two lye droplets from coalescing, stabilizing the emulsion and preventing it from separating into layers of oil and lye. The layer of soap can be seen under a microscope using polarized light. Under these conditions, non-crystalline materials like oil and lye appear more or less magenta, while crystalline materials like soap appear as a halo of red and blue. Figure 1 shows a photomicrograph of raw soap at a magnification of 200X. It was one of thousands of images taken by my student, Avery Moncure, for his honors research project in 2011. The details may be found at http://cavemanchemistry.com/HsmgJojoba2011.pdf, and a time-lapse video is available at https://www.youtube.com/watch?v=bYbWyDMbchI.
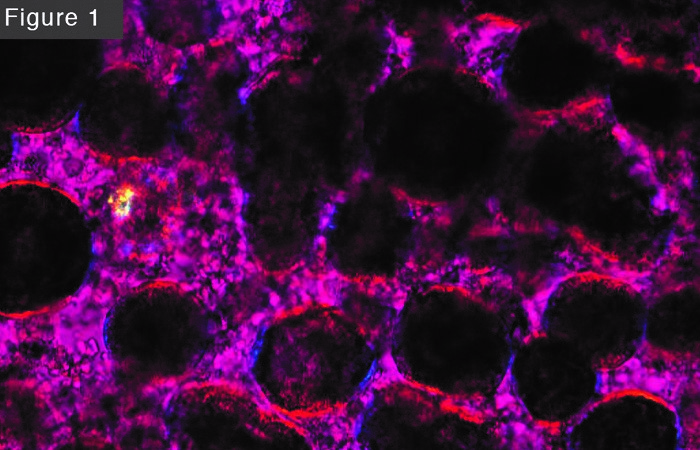
The initial emulsion consists of droplets of one liquid suspended in another, and these droplets flow freely past one another. But as the layer of solid soap forms on the surface, the lye droplets do not slide past one another as easily, with the result that the emulsion becomes thicker, more viscous. This is what is responsible for the trace phenomenon. The emulsion soon becomes so viscous that you can draw on the surface. That's trace.
False trace happens when raw soap thickens without forming a stable emulsion. Most often, it happens when cold lye is poured into cool oil containing fats with relatively high melting points. When you melt solid fats, they start out as translucent fatty solids, but melt into a transparent liquid, which looks just like vegetable oil, even when it cools to room temperature. But anyone who master batches their oil knows that given time, the solid fats will once again solidify, forming lumps of translucent solid fat suspended in liquid oil.
When you pour cold lye into cool oil, any high-melting fats will solidify on the surface of the cool lye droplets. The solid layer of fat has the same effect on viscosity as a solid layer of soap—the lye droplets do not easily slide past one another, and the emulsion thickens. But the fat layer does not stabilize the emulsion as soap does. As saponification proceeds and the soap heats up, the solid fat may re-melt, and having lost their solid surfaces, the lye droplets may coalesce into larger and larger droplets. In other words, the soap may separate into oil and lye.
All of these things are happening simultaneously in a false-trace soap. Solid fat forms at the surface of cold lye droplets, but soap is forming there as well. If there is lots of soap and a little fat, the emulsion may be stable. But if there is lots of fat and only a little soap, the emulsion may separate when it warms above the melting point of the fat. So false trace is a crapshoot. It may or may not cause separation.
Since it may be difficult to distinguish a true trace from a false one in the heat of the moment, the safest course is to avoid the circumstances that lead to false trace in the first place. The popular wisdom used to hold that lye and oil had to be at exactly the same temperature upon mixing. While that would avoid a cold lye/cool oil situation, it is unnecessarily tedious. All that really matters is that the oil should be warm enough to prevent solid fats from “freezing.” At Hamdpen-Sydney, for example, we usually prepare lye days or weeks in advance of a soapmaking session, and use it at room temperature. We control the initial soap temperature by controlling the oil temperature. The exact temperature needed depends on the melting points of any high-melting fats in the formula. Make a habit of recording your oil temperature in each batch record, and if you run into a separation issue, simply increase the oil temperature for the next batch of the same formula. By this simple practice, you can avoid separation issues caused by false trace.
Editorial notes:
Figure1.jpg A stable emulsion of dark lye droplets surrounded by oil. Each droplet is surrounded by a layer of soap, seen under polarized light as a halo of red and blue.
 |
| Buy Supplies - Related Items |
|
Questions & Answers (2) |
 |
| Question + Answer. Here you can ask or answer questions All questions are queued and answered quickly during business hrs. You can also answer questions posted by other customers if you have valuable information. |
 |
2 Question(s) Shown Below |
No items listed at this time, please check back often as we are adding kits/items everyday.
|
|
|
|
|
|
|