| |
|
|
|
|
|
| Waiting For A Cure: Is It Worth The Weight? |
Author: Kevin Dunn
Friday, March 2, 2018 |
|
In advice to a Young Tradesman (1748), Benjamin Franklin penned the oft-repeated proverb, “Time is Money.” His father, Josiah Franklin, was a maker of candles and soap, and young Ben was brought up in that trade. If he wasn’t writing specifically about soap making, it was certainly part of his early business training.
There are many demands of both time and money in the soap making process. Oils, lye, molds, fragrances, colorants and packaging all come with a bill. Weighing, measuring, mixing, pouring, cuttingand packaging all take time. But the single most time consuming part of the whole operation is curing, which may last weeks or even months. Curing takes not only time, but space. The premises we buy or rent must be sufficient, not only for making, packaging, shipping and selling soap, but also for letting it sit, apparently idle, in the saponific equivalent of watching paint dry.
And so in the interest of both time and money, it would be useful to understand exactly what is going on during the curing period and how long it should take.
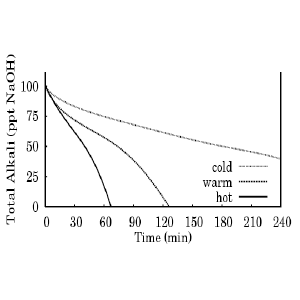
Many people believe that it takes weeks or months for oil to turn completely into soap. My students (Drake Huyzek and William Eskridge) and I studied this in some detail. Figure 1 shows the percentage of alkali (NaOH) remaining in three soaps as a function of time. “Cold” soap was kept at a constant temperature of 50ºC (122ºF). “Warm” and “hot” soap was kept at 60ºC (140ºF) and 70ºC (158ºF), respectively. The hot and warm soaps were completely saponified in one or two hours. The cold soap was half-done in four hours, and extrapolating this data we would expect it to be completely done in eight hours. Cold process soap does not maintain a constant temperature; it starts cold, gets hot, and cools down again. But given the rate of saponification for even cold soap, it is reasonable to suppose that cold process soaps are completely saponified in hours, not weeks.
This expectation has been ratified many times in our work. We typically test the alkalinity the day after a soap was made, and it rarely fails the “zing” test. For those who like primitive methods, you simply touch the soap with a wet finger, touch the finger to your tongue, and note the presence or absence of something vaguely like a mild electric shock, zap, or zing. A more modern test is to place a drop of phenolphthalein indicator (dissolved in alcohol, not water) onto a soap and note the presence or absence of a pink color. Soap that does not zing the tongue or turn phenolphthalein pink contains less than 1 part per thousand (1 ppt, 0.1%) residual NaOH. This is the standard in the commodity soap industry.
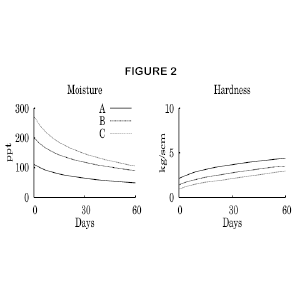
Day-old soap that passes these tests is perfectly safe to use. So why the long cure period? Figure 2 shows what happens to the moisture content and hardness of cold process soap over a 60 day period. Soap A was a low-water soap, made with lye at a concentration of 50% NaOH. Soaps B and C were made with lye at 33% and 25% NaOH, respectively. Two things happen simultaneously as the soap ages. The moisture content falls as water evaporates from the soap, and hardness increases.
Moisture and hardness? How do you measure those? I followed moisture by weighing the soap every few days. As water evaporated, the weight decreased. And since I know exactly how much water was in the soap to begin with, I was able to calculate the remaining moisture. Soap B, for example, started at 201 ppt (20.1%) moisture and wound up 60 days later at 89 ppt (8.9%) moisture. The details of calculating moisture from weight are given in Scientific Soapmaking (Dunn, 2010), Chapter 14.
I measured hardness using a device invented for soil evaluation. The soil penetrometer is a spring-loaded gizmo about the size of a screwdriver. You push the point of the penetrometer into a bar of soap until the tip is buried to a depth of 1/4”. The hardness can then be read off the scale on the side.
In addition to moisture content and hardness, one other property of soap changes as it cures. While the bulk of the NaOH is consumed by the saponification reaction in the first day, a tiny amount remains. At 1 ppt or less, it is not detectable by the tongue and does not turn phenolphthalein indicator pink. Nevertheless, carbon dioxide in the air reacts with whatever NaOH is left and converts it to sodium carbonate. After curing, the soap is slightly less alkaline than it was the day it was taken from the mold. While fresh soap is not harmful, it is possible that some consumers may perceive cured soap as slightly milder.
If soap is safe to use out of the mold, why do we bother with curing? After all, the changes that occur will do so just as well in a customer’s bathroom as on our curing racks. The most important reason for curing is commercial, not scientific. Soap is sold by weight, and United States law requires that the weight be listed on the package. As soap cures, its weight decreases. So if you package a fresh bar at 4 ounces, and if it remains unsold for any length of time, its weight will fall below the labeled weight by the time it is sold. The product is then considered misbranded.
How long does it take for soap to reach a stable weight? It depends on how much moisture was present initially, the temperature and relative humidity of the curing room, and the size of the bar. The easiest and most reliable way to tell whether your soap has cured long enough is to select one bar from each batch as the designated weigher. Record the weight of this bar on your batch sheet from week to week until the weight stops falling. Even then, it is wise to include a safety factor when labeling the weight. You might, for example, label as 4 ounces only those bars that weigh at least 4.2 ounces. This will account for any remaining moisture that may evaporate after packaging. By curing your soap only for as long as necessary, you will move your soap through the curing process at an appropriate pace, thus saving both time and money.
 |
| Buy Supplies - Related Items |
|
Questions & Answers (1) |
 |
| Question + Answer. Here you can ask or answer questions All questions are queued and answered quickly during business hrs. You can also answer questions posted by other customers if you have valuable information. |
 |
1 Question(s) Shown Below |
No items listed at this time, please check back often as we are adding kits/items everyday.
|
|
|
|
|
|
|